Isotopes
As in the previous material explained, atomic mass of the element is the sum of the masses of all of the protons and all of the neutrons in one atom of the element. Each of these particles weigh 1 atomic mass unit. Electrons have a much lower mass than protons or neutrons, and so their mass can be ignored when determining atomic mass.
If we look at one atom of 12C, we see that if it has 6 protons and 6 neutrons, and so the atomic mass of this atom should be 12 and not 12.011 as is reported on the periodic table. However, the average atomic mass of carbon is not exactly twelve because it has isotopes – these are elements that have the same number of protons, but a different number of neutrons. One example is bellow shown for carbon:
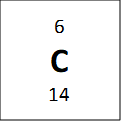
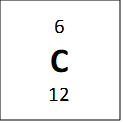
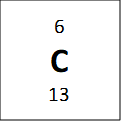
All of these atoms are carbon, because they all have 6 protons, but they have different masses. The average atomic mass of 12.011 amu comes from a weighted average of the masses of the isotopes, weighted by the percent composition of a natural carbon sample (98.9% 12C, 1.1% 13C and a negligible amount of 14C) and so if we take percentages into account we get the following equation for mass:
12 × 0.989 + 13 × 0.011 = 11.868 + 0.143 = 12.011