The ideal gas equation relates the properties of an ideal gas to each other. These properties are pressure (P), volume (V), a number of moles (n), and temperature (T). The ideal gas equation is given below.
PV = nRT
The variable R is the universal gas constant. Its numerical value will depend on the units of the other variables. There are two commonly used values, R = 0.08206 L atm mol-1 K-1 and R = 8.314 J mol-1 K-1. All temperatures should be in units of Kelvin (K) when performing calculations dealing with gases. Additionally, the ideal gas equation can be used to calculate the density (d) or molar mass (M) of an ideal gas. The relevant equations are given below.
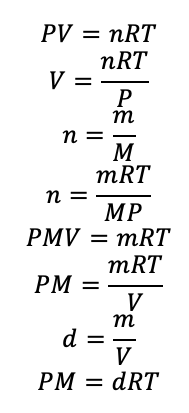
It should be stated that the ideal gas equation is only an approximation of real gases and values calculated by these methods will not be totally accurate.